BAYOUSIDE CLASSROOM WATER SAMPLING PROGRAM
WHAT IS WATER QUALITY?
The term “water quality” refers to the chemical and physical properties of a given body of water. Environmental scientists are often concerned with the temperature, salinity, or presence of dissolved substances in natural waters because of their effect on animals and plants. These aspects of water quality are also of great importance to community leaders because of our reliance on water for drinking, food production (fisheries and farming), transportation, and industry. For example, approximately 52% of the land area of Terrebonne Parish (in which LUMCON is located) is covered with water, 70% of the workforce is employed in the maritime industry (mineral and oil exploration, ship building, fishing, etc.), and the annual fishery revenue is over $65,000,000. With such strong ties culturally to the bayous, marshes, and Gulf of Mexico, water quality is a prime concern for our citizens.
LLUMCON’s Estuarine Environment
LUMCON is located in the Barataria-Terrebonne Estuary, an area bordered by the Mississippi River to the north and east, the Atchafalaya River to the west, and the Gulf of Mexico to the South. Estuaries mark the boundary between the land and the sea; the place where freshwater mixes with the salty ocean. Because it is an area of mixing, the estuary is defined by a gradient of water quality (e.g. temperature, salinity, dissolved gases).
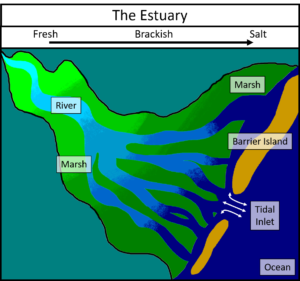
The most landward portion of the estuary receives input from rain, surface runoff, and discharge from lakes and rivers. Water quality in this region is most similar to the water sources that supply it. The plants and animals here are adapted to a freshwater lifestyle and cannot tolerate dissolved salt in their water supply.
As water flows through the estuary, it gradually mixes with saltier water moving in from the ocean. Thus, as you move from the land to the sea, water quality steadily changes until it matches that of the open ocean. At the most oceanward end of the estuary, the plants and animals are adapted to life in saltwater.
In the estuary, you will encounter water quality characteristics that fall in between freshwater and saltwater. Estuaries are also unique because tidal forces influence them. Tides are the regular rise and fall of water in the ocean caused by the gravitational pull of the sun and moon. At high tide, the ocean pushes into the estuary and saltwater moves closer to the land. At low tide, the estuary drains and the ocean is pushed back to be replaced by freshwater flowing from the land. These regular movements of ocean water into and out of the estuary cause the salinity at any given location within the estuary to continually change. Estuarine organisms, therefore, must be able to tolerate a variety of water quality conditions.
In addition to the tides, other natural disturbances can affect how far saltwater moves up the estuary. For example, heavy rains upstream from the estuary will increase the flow of freshwater into the estuary and push the saltier water further towards the ocean. In contrast, during drought conditions, freshwater flow is diminished and saltwater can push very far inland. In fact, during the drought of 1999-2000, saltwater was detected as far inland as Houma. Human activities can also affect the progression of saltwater into the estuary. For instance, the dredging of canals for navigation or petroleum exploration (pipeline canals) gives saltwater a direct route inland. Because these canals are usually deeper than natural bayous, saltwater, which is heavier than freshwater, slowly moves inland along the bottom of the canal. This “saltwater intrusion” is a primary cause for the death of freshwater plants such as our native cypress.
BAYOUSIDE CLASSROOM PARAMETERS
To help you understand water quality and how it is measured, you will be collecting and analyzing water samples from several areas surrounding LUMCON. We will provide you with the tools needed to collect your samples and to measure several water quality parameters. Once you are finished, you will enter your data into a database located on the Internet. This will allow you to access your data (and that of other students) all year to see how water quality changes with the season. The five (5) parameters you will be measuring are listed below.
- TEMPERATURE
- SALINITY
- DISSOLVED OXYGEN
- PH
- WATER CLARITY/TURBIDITY
