HYPOXIA
WHAT IS HYPOXIA?
Hypoxia is a phenomenon that occurs in both freshwater and saltwater aquatic systems where the dissolved oxygen concentration drops below a certain point and begins to adversely affect respiring organisms. Generally speaking, the dissolved oxygen concentration during an episode of hypoxia is below 2ppm. Anoxia occurs when dissolved oxygen levels drop to zero.
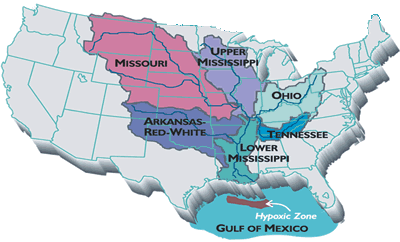
Low dissolved O2 concentrations have been recorded in inland freshwater ponds as well as coastal areas around the world. According to the United Nations Environment Programme, in 2003 there were 146 instances of coastal hypoxia worldwide. These areas are shown on the map below.
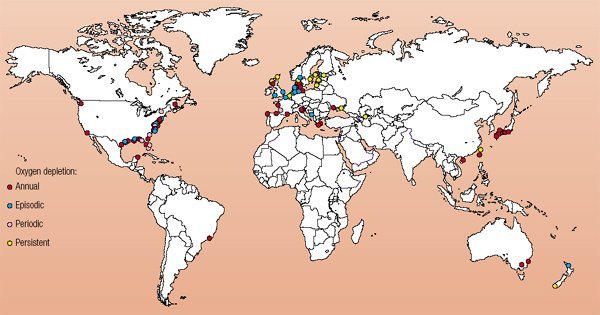 |
| Source: United Nations Environment Programme, GEO Yearbook 2003 (Nairobi: 2004), compiled from Boesch 2002, Caddy 2000, Diaz et al. (in press), Green and Short 2003, Rabalais 2002. |
As the map indicates, hypoxia can occur with varying frequency. Most of these cases happen on an annual basis, usually in the warmer months of the year.
WHAT CAUSES HYPOXIA?
There are many factors that contribute creating a hypoxic area. Temperature, salinity, vegetation, and bacteria are just a few of these.
Before these factors are discussed, an understanding of how the oxygen gets into the water is needed. Water holds much less life-giving oxygen than air. Therefore, oxygen levels are extremely important when dealing with aquatic systems. Because oxygen is a byproduct of photosynthesis, underwater plants and algae are the major source of oxygen in aquatic habitats. Another way oxygen gets into the water is through the air-water interface. Since there is more oxygen in the air than the water, the oxygen has a natural tendency to diffuse into water. This process is enhanced by windy conditions, especially during storms. Actively mixing the water to maximize air-to-water contact also helps to add oxygen. This is done, for example, by adding splashing fountains in ponds.
Now that we have established how oxygen can get into the water, lets look at how environmental factors can alter dissolved oxygen concentrations.
Temperature
Temperature has a very important effect on dissolved oxygen. As the temperature of the water goes up, the water loses its ability to hold dissolved oxygen and the concentration of dissolved oxygen goes down. When the water cools, it regains the ability to hold higher amounts of oxygen. The chart below simplifies this rule.
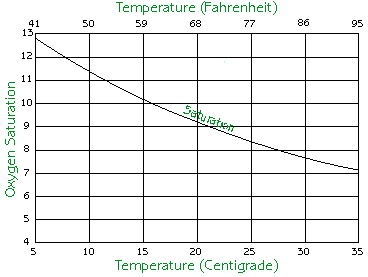 |
| Source: Koi Club of San Diego |
Knowing this relationship, one can deduce that hypoxia tends to occur in the warmer months of the year, namely during the summer.
Salinity
Salinity (the amount of dissolved salts in the water) has a very interesting affect on the amount of oxygen in the water. First, there is an inverse relationship between the amount of salt versus the amount of dissolved oxygen in the water. 5 grams of salt in 1000 grams of water (5 ppt) will decrease the oxygen saturation levels about 1 mg/l.
Salinity also has another property that helps to create hypoxic zones. Salt water is more dense than fresh water. This causes the two layers of water to form a lighter layer of fresh water on the top of a heavier layer of salt water on the bottom. This prevents adequate mixing of the water column and does not allow oxygenated water to get to the lower depths. Therefore the heavier, saltier layer at the bottom may become oxygen-depleted.
Most ponds and lakes are freshwater and are free from these salinity considerations.
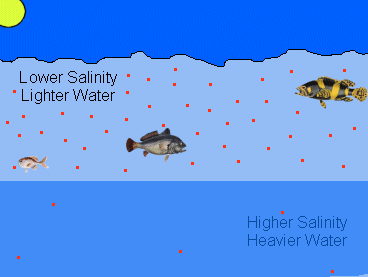 |
| This image shows how salinity can affect hypoxia. The red dots represent the relative concentration of dissolved oxygen during a hypoxic event. Notice that due to inadequate mixing, the concentration of oxygen in the saltier bottom layer of water is lower. |
Vegetation
Vegetation adds oxygen to the water. Plants undergo photosynthesis and release oxygen, so all aquatic plants play an integral role in maintaining healthy levels of oxygen. Also, remember that phytoplankton use photosynthesis, too, and add oxygen to the water. Even though most phytoplankton species are microscopic, these organisms are extremely important.
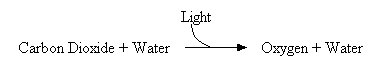 |
| This is a simplified schematic of photosynthesis. Note how oxygen is released as a byproduct of the reaction. |
Bacteria
Bacteria can have a devastating effect on the amount of dissolved oxygen. Bacteria feed on decaying material as it sinks to the bottom. These bacteria respire and use oxygen just like fish. So with billions and billions of bacteria respiring, the dissolved oxygen level may dramatically drop to dangerous levels. Bacteria play an integral role in the hypoxic zone that occurs each summer off of the coast of Louisiana.
WHY IS HYPOXIA BAD?
Zones of low oxygen cause devastating effects on the fauna that depend on oxygen. Some animals like fish and crabs can move away from a hypoxic area to a more suitable location. Animals like bivalves (clams and oysters) can not always escape the deadly effects of hypoxia. These animals often perish in such conditions.
In closed systems like ponds, the animals may have no way out of a low oxygen zone. Some species in deep-water may not be able to move from the lower layers of the water where the low oxygen areas tend to occur. This is because of the temperature or pressure needs of the organism. These animals are effectively trapped in this location and may die.
Fish Kills
In some cases, hypoxia may cause a fish kill. When hypoxia takes hold of an entire pond or on a vast area of open ocean, the animals cannot escape and many of them die. Fish kills are detected by seeing dead, floating fish and other animals. The observer may also notice stressed fish at the water surface gulping for life-saving oxygen. These fish often perish as well. Bacteria will begin to decompose these dead fish and lower dissolved oxygen levels even further. Eventually the fish will decompose or will be eaten by scavengers and the oxygen will slowly return to a more acceptable level. Unfortunately, a fish kill may lead to an unbalanced fish population and other environmental problems.
Floating fish were observed by LUMCON employees on Monday August 23, 2004. Upon further inspection, we concluded that the pond’s dissolved oxygen dropped to dangerously low levels and caused a fish kill. Our findings are in the chart below.
| August 19, 2004 | August 23, 2004 | August 25, 2004 | |
| Bottom DO Reading | 1.8 | 0.4 | 2.8 |
| Top DO Reading | 3.4 | 0.6 | 5.8 |
| This chart shows how the oxygen dropped on August 23 to dangerously low levels, causing a fish kill. The DO measurements before and after this event are given for comparison | |||
These images were taken after the fish kill at LUMCON.
 |
 |
| These fish were victims of hypoxia. | Hypoxia has also killed these fish. |
 |
 |
| This crab left the low concentrations of oxygen in the water to get some life-saving O2 from the atmosphere. | Lots of shorebirds show up during fish kills to catch an easy meal. |
The Dead Zone
Every summer, a very large area of hypoxia forms off the coast of Louisiana. It is commonly known as The Dead Zone. LUMCON researcher Nancy Rabalais maps this area and compares it to data she has been collecting since the mid-1980’s.
The mechanics of hypoxia in the Gulf of Mexico is somewhat complex. Fortunately, it can be simplified to just a few processes. The story of the dead zone does not start anywhere near Louisiana; it starts in the largest watershed in the United States: the Mississippi River watershed. Excess nitrogen and other nutrients used by midwest farmers wash away from their fields and into the tributaries of the Mississippi River. Over time, all of these nutrients flush down the Mississippi and are deposited into the gulf. Once the nutrients get to the gulf, the phytoplankton devour the nutrients and multiply. Remember that phytoplankton use photosynthesis, which produces oxygen, so at this point, the oxygen is not yet depleted. Once the phytoplankton begin to run out of nutrients, there is a mass die-off of the tiny plants and they begin to fall to the gulf floor. This is where hypoxia begins. Bacteria decompose the falling phytoplankton and use enormous amounts of oxygen. If you couple this cycle along with the warm temperatures and salinity effects mentioned earlier, it creates a huge area of depleted oxygen concentrations.
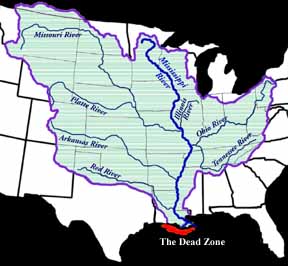 |
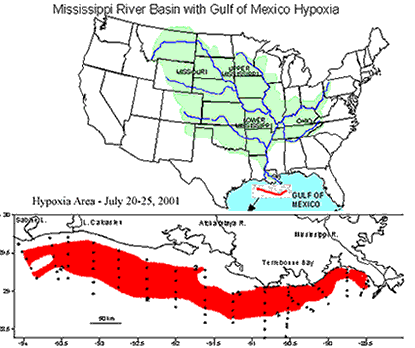 |
| This is the Mississippi River watershed. Source: National Source for Appropriate Technology | This map indicates how widespread the area of hypoxia was in the summer of 2001. It was more than 7722 square miles in area. Source: NOAA |
The topic of hypoxia is actually quite controversial. Agriculturalists in the midwest do not believe that their industry is the sole culprit for the existence of the dead zone. In fact, they sometimes say the term ‘dead zone’ is misleading and misrepresents the truth. Scientists, on the other hand, tend to blame the nitrates that empty into the gulf from farmers. There has been legislation regarding this topic (see The Harmful Algal Bloom and Hypoxia Research Amendments Act of 2003 [H.R. 1856 IH]).


