DISSOLVED OXYGEN
First devised in 1889, the Winkler method is considered the “gold standard” for measuring the concentration of dissolved oxygen in a sample of water. Through a series of chemical reactions, the O2 combines with iodine to form a golden yellow chemical. Therefore, each oxygen molecule is associated with an iodine molecule, and we can measure oxygen by measuring the iodine. When the iodine is neutralized by the addition of sodium thiosulfate, the golden color disappears and we can determine how much iodine (hence oxygen) was in the sample. (Note: some oxygen test kits use a starch indicator that turns the iodine solution from yellow to a deep blue color to make it easier to distinguish the color change.)
Once the water sample is collected, it is important to “fix” the sample immediately. Phytoplankton, bacteria, and other organisms in the sample can quickly change the oxygen content of the sample through photosynthesis and respiration. The first step of the Winkler method is the addition of manganous sulfate (a source of manganese ions) to the sample, quickly followed by the addition of lithium hydroxide (a strong base) and potassium iodide (a source of iodine). In the presence of the strong base, each oxygen atom binds with a manganese ion to form a manganous hydroxide complex. This reaction creates a pale precipitate that will eventually sink to the bottom of the sample container. Sulfuric or sulfamic acid is added to the solution to reduce the pH and dissolve the precipitate. When this occurs, free iodine is produced at a rate of one iodine molecule per manganese ion. This produces one iodine molecule for each oxygen molecule in the sample. At this point, the sample is “fixed” (all the oxygen converted to iodine) and can be set aside for several hours before final analysis.
The final step of the dissolved oxygen measurement is a titration. Titration is a method of determining the concentration of a substance in a solution by adding a second chemical of a known concentration to produce a controlled chemical reaction. In the titration step, sodium thiosulfate is slowly added to the solution until all the iodine is neutralized (color disappears). We can determine how much iodine was in the solution from the amount of thiosulfate added. Furthermore, because each iodine molecule was produced by the reaction of a single oxygen atom, the amount of thiosulfate added also tells us how much oxygen was in the sample.
STEPS IN THE WINKLER METHOD
1. Manganese(II) ions liberated from the manganese sulfate are loosely bound with excess hydroxide.
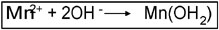
2. Manganese(II) is oxidized to Manganese(III) in the presence of a strong base and binds the dissolved oxygen.
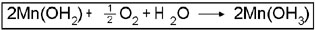
3. Free iodine is produced upon acidification of the sample at a rate of one I2 molecule for each atom of oxygen.

4. Free iodine complexes with excess iodide ions.
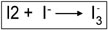
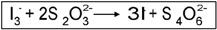
5. The iodine/iodide complex is reduced to iodide with thiosulfate.
From: Grasshoff, K. 1983. Determination of oxygen (chapter 4). In: Grasshoff, K., Ehrhardt, M., Kremling, K. (eds.). Methods of seawater analysis. Second, revised and extended edition. Verlag Chemie, New York.
Sampling Instructions
- Collect water as described here using a clean and thoroughly rinsed container.
- Fill and cap the glass sample bottle ensuring that no air bubbles are trapped inside.
- If using a LUMCON-style sampler, immediately cap the glass bottle located inside the sampler.
- If using an open top sampler, such as a bucket, hold the glass bottle underwater until all air is removed then cap while the bottle is still submerged.
- Put on gloves and safety glasses before proceeding to the next steps. Your procedure will vary with the type of oxygen kit available.
Complete instructions can be found using this link to our Winkler Titration Instructions document.
|
Important steps to remember:
|
